BASIC SCIENCE 2ND TERM: Activity Series Of Metals
WEEK 3
SUBJECT: Basic Science
TOPIC: Activity Series
CLASS: JSS 1
DURATION: Two Periods (40 minutes each)
By the end of the lesson, students should be able to:
I. Define activity series
ii. Carry out a simple experiment to show how metals and non-metals react
iii. Write the activity series of some metals and non-metals correctly
KEY VOCABULARY WORDS
-
Activity
-
Series
-
Reaction
-
Metal
-
Non-metal
-
Reactivity
-
Chemical change
RESOURCES AND MATERIALS
-
Iron nail
-
Copper wire
-
Zinc piece
-
Sulphur powder
-
Water
-
Salt solution
-
Test tubes or transparent cups
-
Chart showing activity series
BUILDING BACKGROUND
The teacher asks students:
-
Have you noticed how iron rusts when exposed to water?
-
Why do some metals react faster than others?
The teacher explains that some substances react more easily than others, and this helps us arrange them in a special order called the activity series.
CONTENT
Meaning of Activity Series
The activity series is a list of metals and non-metals arranged in order of their reactivity with other substances.
Some substances react very fast, while others react slowly.
What is Activity Series?
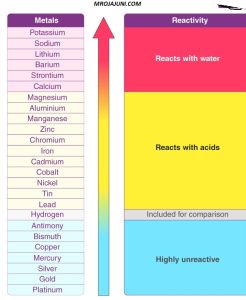
The activity series of metals is a list that ranks metals in order of their reactivity. This series is useful in predicting the outcome of displacement reactions, where a more reactive metal can displace a less reactive metal from its compound. The general rule is that a metal higher in the activity series can replace a metal lower in the series from its compound. BASIC SCIENCE 2ND TERM: Activity Series Of Metals
Reactivity is the ability of a substance (metal or non-metal) to react or change when it comes in contact with another substance.
In simple words:
Reactivity means how fast or easily a substance reacts.
Examples:
-
Zinc reacts quickly with acid → it is highly reactive
-
Gold hardly reacts with anything → it is low in reactivity
So, the higher the reactivity, the faster the chemical reaction happens.
Here is a simplified activity series of metals, listed in descending order of reactivity:
- Potassium (K)
- Sodium (Na)
- Calcium (Ca)
- Magnesium (Mg)
- Aluminum (Al)
- Zinc (Zn)
- Iron (Fe)
- Nickel (Ni)
- Tin (Sn)
- Lead (Pb)
- Hydrogen (H)
- Copper (Cu)
- Mercury (Hg)
- Silver (Ag)
- Gold (Au)
- Platinum (Pt)
The metals at the top of the activity series are more reactive, meaning they have a greater tendency to lose electrons and form positive ions. Metals towards the bottom of the series are less reactive. Hydrogen is included in the series because it can act as a metal in certain reactions.
BASIC SCIENCE 2ND TERM: Activity Series Of Metals
Keep in mind that this is a generalization, and the reactivity of a metal can also depend on specific conditions and the nature of the reaction. For example, the reactivity of metals can change in different environments or when reacting with different substances.
Important Uses of the Reactivity Series in Predicting Chemical Reactions
The reactivity series of metals is a crucial tool in understanding the properties and behaviors of metals, providing valuable insights into their reactivity. Beyond its theoretical applications, the reactivity series finds practical use in predicting and understanding various chemical reactions involving metals. Here are some important applications of the reactivity series:
1. Prediction of Reactions with Water: The reactivity series aids in predicting the outcomes of reactions between metals and water. Metals that are more reactive than hydrogen, such as potassium and sodium, can react with cold water to form hydroxides and liberate hydrogen gas. The chemical equation for the reaction between potassium and water is represented as follows:
This application allows us to foresee the behavior of metals when exposed to water.
2. Understanding Reactions with Acids: Metals above hydrogen in the reactivity series can form salts when reacting with hydrochloric acid or sulfuric acid. The liberation of hydrogen gas is also a common feature of these reactions. For instance, the reaction between zinc and sulfuric acid produces zinc sulfate and hydrogen gas, expressed by the equation:
The reactivity series thus serves as a tool to anticipate the outcomes of metal-acid reactions.
3. Prediction of Single Displacement Reactions: The reactivity series is invaluable in predicting single displacement reactions between metals. Higher-ranking metals can displace lower-ranking metals in such reactions. An example is the displacement of copper from copper sulfate by zinc, as illustrated by the equation:
This concept has practical applications in the extraction of metals, such as the extraction of titanium from titanium tetrachloride using a single displacement reaction with magnesium.
In summary, the reactivity series of metals is not merely a theoretical construct but a practical tool with significant applications in predicting and understanding chemical reactions involving metals. From reactions with water and acids to single displacement reactions, the reactivity series provides a framework for anticipating the behavior of metals in various chemical contexts.






